
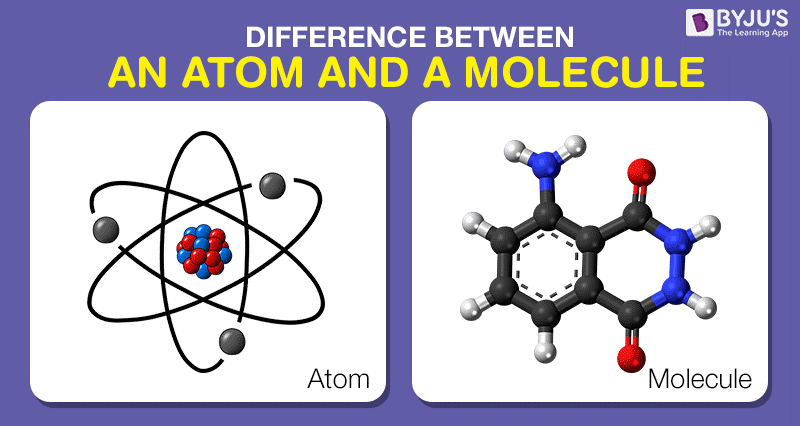

Therefore, the key difference between molecule and atom is that a molecule is a combination of two or more atoms via chemical boding whereas an atom is an individual chemical species which can combine with each other to form molecules and ions. The below infographic provides more facts on the difference between molecule and atom. Moreover, based on the existence, the difference between molecule and atom is that the molecule has a stable existence whereas, the individual atoms are unstable. A single atom cannot exist independently except for noble gases while molecules exist independently because they have low energy.
DIFFERENCE BETWEEN ATOM AND MOLECULE IN TABULAR FORM PLUS
Hence, the chemical formula of a molecule has a set of English letters along with the number and some other symbols such as parentheses, dashes, brackets, and plus (+) and minus (−) signs. However, for atoms, an English letter shows the symbol of a chemical element to which the atom belongs. Accordingly, a chemical formula shows the symbols of atoms present in the molecule.

Moreover, we can use chemical symbols to represent a molecule we name it as a chemical formula. Hence, the key difference between molecule and atom is that a molecule is a combination of two or more atoms via chemical bonding whereas an atom is an individual chemical species which can combine with each other to form molecules and ions. What is the Difference Between Molecule and Atom? Protons, neutrons and electrons are subatomic particles of an atom. This nucleus is surrounded by a cloud of electrons. It consists of an atomic nucleus containing protons and neutrons.

An atom is extremely small the size is around 100 pm. A chemical element is a species of atoms thus, atoms have both chemical and physical properties of its particular chemical element. What is an Atom?Īn atom is the smallest repeating unit that makes up all matter. Some molecules have a symmetrical geometry while other have non-symmetrical geometries. It thus gives the bond lengths and bond angles between these atoms. Consequently, the geometry displays the spatial arrangement of atoms. Moreover, we can use a structural formula that gives the arrangement of atoms in the molecule.ĭifferent molecules have different geometries. We can calculate the molecular mass of the molecule using this chemical formula. Sometimes other than the chemical symbol of atoms and numbers, we use some other symbols as well parentheses, dashes, brackets, and plus (+) and minus (−) signs. The chemical formula is a set of symbols that give the atoms and the ratios between them that has combines to form the molecule. To understand the atoms present in a molecule, we can use its chemical formula. This forms cations (positively charged ions) and anions (negatively charged ions) which are held together by electrostatic attraction forces or ionic bonds.įigure 01: Formation of a Hydrogen Molecule Ionic bonds form when the electrons completely exchange between atoms. A covalent chemical bond forms when atoms share their electrons with each other to complete the octet of electron configuration. The atoms in a molecule can bind with each other via covalent bonds or ionic bonds. A heteronuclear molecule forms when atoms of different chemical elements combine with each other. A homonuclear molecule forms when atoms of the same chemical element make up the molecule. These atoms can be of the same chemical element or different chemical elements. Side by Side Comparison – Molecule vs Atom in Tabular FormĪ molecule is a group of atoms that combine with each other via chemical bonds. It then becomes a molecule, and we can represent it by the chemical formula O 2. However, this atom of oxygen does not exist independently, and it is only when it combines chemically with another atom of oxygen that it becomes stable. For example, the smallest unit of oxygen gas is the atom of oxygen we can represent it by letter O. A chemical element is a species of atoms. In layman’s terms, we can say that the most basic and also the smallest unit of any chemical element is an atom. The key difference between molecule and atom is that molecule is a combination of two or more atoms via chemical bonding whereas atom is an individual chemical species that can combine with each other to form molecules and ions.Īll matter in the world is made up of atoms and molecules hence we can consider them as the building blocks of everything on this planet, including us.


 0 kommentar(er)
0 kommentar(er)
